Scientific Publications

Insulin Glargine: A Long-Acting Solution for Type 1 & Type 2 Diabetes Management
Assessing PK, PD, Safety & Tolerability for Improved Glycaemic Control

Peptide Analysis: Case Study
Insights into Veeda’s Proficiency in Analyzing Peptide Molecules with Precision

505(b)(2) Pathway: Approval for Three Different Formulations
Evaluating the Safety and Efficacy of Three Different Formulations of Loperamide Hydrochloride

Leuprolide Acetate 30 mg Case Study
Leuprolide Acetate Pharmacokinetic Case Study in Prostate Cancer

Risperidone 25mg injection Case Study
Assessing Bioequivalence of Risperidone Long-Acting Injectable in Patients with Schizophrenia

Navigating Complexities of Inhalation Clinical Studies
A Comparative Bioavailability Study of Tiotropium Bromide Inhalation Solution for COPD Management
Bioequivalence Assessment of Iron Sucrose Injection
Analysis of Efficacy and Safety for Anaemia Management in Healthy Volunteers
Scientific Briefing on Higher-Order Structure Assessment: A Critical Step in Biosimilar Development

Paliperidone Pharmacokinetic Bioequivalence Study
Managing the BE Study Challenges of an Antipsychotic LAI
BE (Bioequivalence) Study of Oncology drug candidate
Managing Complexities associated with BE (Bioequivalence) Study of Oncology drug candidate
Bioequivalence of Brinzolamide & Brimonidine
Evaluating the Bioequivalence of Brinzolamide & Brimonidine for the treatment of Open-angle Glaucoma
Euglycemic Clamp Study
Comparative evaluation of Pharmacokinetic & Pharmacodynamic properties to assess Insulin Action for Management of Diabetes
Functional Characterization of Abatacept Biosimilar
Veeda’s successful execution of Pharmacodynamic Characterization of Biosimilar to initiate Phase I clinical trials for the treatment of Rheumatoid Arthritis

Pharmacokinetic Bioequivalence Study
Veeda’s successful execution of two-way crossover PK study of Inhalation powder in healthy volunteers

Dermatology Phase I Study
Veeda’s successful execution of a Phase I, double blinded SAD and MAD study in healthy volunteers treating Vitiligo

First to File Study
How we delivered Quality Results for a leading US-based Pharmaceutical Brand in a record period of 12 days
Fresh / Relapsed Multiple Myeloma patients
How we helped an Indian Multinational Pharmaceutical Company reduce Screen Failure Ration significantly

Bioanalytical Aspects of Multiple Myeloma Patient Trial Study
A Multinational pharmaceutical company that specializes in development, manufacturing, and distribution
Quetiapine Fumarate Prolonged Release Tablets
An Indian multinational pharmaceutical company was planning the marketing approval in the EU for Schizophrenic patients.
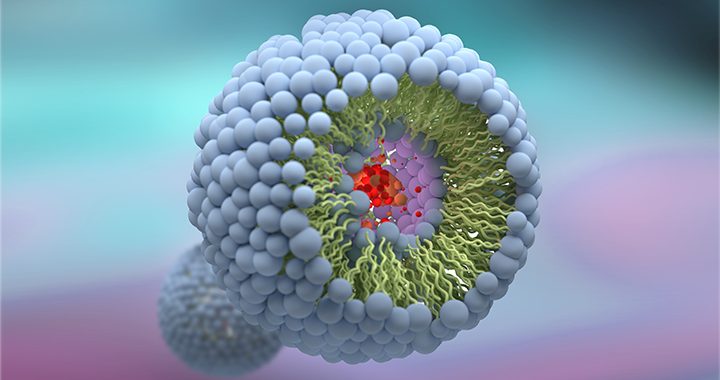
Handling a complex liposomal molecule – Amphotericin B
A specialty pharmaceutical company based in USA that specializes in development, manufacturing, and Commercialization

Whitepaper on Complex Generics and 505(B)(2) Regulatory Pathways
Gain insights into our capabilities in supporting 505(b)(2) applications and complex generics development


