Development and Execution of In Vivo Bioassays
January 8, 2022
Home > Veeda Insights > Data Integrity in Clinical Research

The scientific community has been a witness to some of the worst tragedies in the history of clinical trials data integrity. From the year 2015 till date, The Journal of the American Medical Association (JAMA) and the JAMA network journals have published at least 18 notices citing concern over data error and/or falsification of data.1 For instance, the trials conducted by a Japanese anesthesiologist and researcher, to treat post-operative nausea and vomiting, were reviewed by the Japanese Society of Anesthesiologists (JSA) in the year 2012 to find startling revelations. The data obtained from the trials were either totally fabricated or fraudulent and approximately 210 papers published by the anesthesiologist had falsified data.2 Lapses in data integrity causes significant loss of revenue with the direct costs estimated to be close to 525,000 US dollars while indirect costs amounting to approximately 1.3 million US dollars.3
Such scientific misconducts served as a wake-up call to tighten regulations and laws to monitor drug development and drug use. Scientists acknowledged the need for data integrity at every stage to safeguard human subjects, starting from pre-clinical development to pharmacovigilance.
What is Data integrity?
Data integrity is defined as paper-based or electronic data that is complete, accurate, consistent, and reliable through its lifecycle from the time of data creation, archival, scanning, retention, and destruction.4 The updated International Council for Harmonization Guideline for Good Clinical Practice (ICH GCP E6[R2]) reiterates the need for data integrity as well as the importance of monitoring clinical data throughout the study.
The United States Food and Drug Administration (FDA) use the ALCOA acronym to define expectations with respect to data integrity.4
Data compliance issues
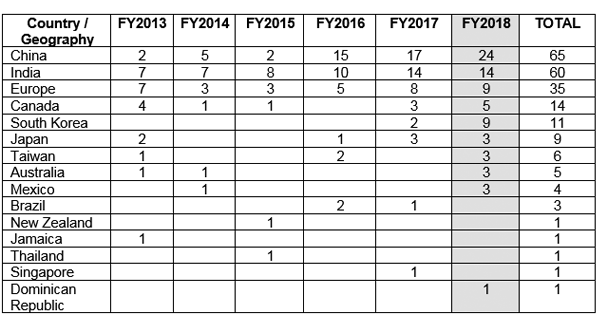
The FDA issued Good manufacturing practices (GMP) warning letters to various countries outside the United States (US) citing compliance issues over data integrity. Figure 1 shows China to have received maximum GMP warning letters followed by India and Europe.5
Figure 1 GMP warning letters issues outside US5
Data integrity can be monitored by keeping a check on the following areas:6
SDV
Strict adherence to good documentation practices (GDP) in clinical trial records is a way to ensure data integrity. GDP should be followed for paper records as well as electronic records and signatures. Equally important is the need to retain and organize essential documents required before the start of a clinical trial, during the trial, and after completion or termination of a trial. The collection of essential documents that is kept at the sponsor site and investigator site is called the clinical trial master file (TMF). TMF plays a major role in facilitating trail conduct and management thereby allowing for data integrity and GCP compliance at all stages of the clinical trial. The TMF is the document that is reviewed during an audit or inspection.8
Many pharmaceutical companies are now moving towards electronic TMF (e-TMF) for easier management of large and complex clinical trials that involve numerous departments or CROs.
Data access and control
It is necessary to exercise caution while handling data from clinical trials. Confidentiality of data should be maintained during all the phases of a clinical trial including interim data results.9 The ability to tamper with data such as changing, deleting, or falsifying data should be restricted by clearly demarcating roles. This also prevents potential conflict of interest between similar roles that may hamper data integrity.4
The National Institute of Health (NIH) states that only voting members of the Data and Safety Monitoring Board (DSMB) should be permitted to look at the interim analyses results unless circumstances makes it necessary to share data, such as in the case of serious adverse events.9 In addition, the DMC members should not have any conflict of interest that would influence the outcome data. The FDA has also recommended the use of an “independent statistician” model to analyze interim data who is independent of the principal investigator and trial sponsor and reports unbiased results to the DMC.10
Data Monitoring
It is necessary to set up an independent data monitoring committee (DMC) that prioritizes the safety and interests of enrolled subjects and scrutinizes authenticity of data as well as the clinical trial conduct.9
On-site monitoring: is carried out to trace any discrepancy between the source data and data entered. It is also particularly useful to see if the site staff is familiar with the study document and if the staff has demonstrated accountability to carry out the trial ethically and responsibly.11
Centralized risk-based approach: ICH GCP E6(R2) emphasizes the need for centralized monitoring to reduce the number of trial visits by the clinical monitor and to allow for remote spotting of reliable and unreliable data by statisticians or other data management staff.4,11
Risk-based monitoring: The sponsor company is required to develop a robust risk management plan to prevent or mitigate any risk to human subjects by overseeing trial conduct and monitoring data quality across trial sites.11
Data integrity Audits12
To avoid huge financial repercussions and loss of business, sponsor companies and CRO should lay sufficient emphasis on maintaining data integrity at every step of the clinical study for its completeness, accuracy, and consistency.
Sources
1.Bauchner H, Fontanarosa Phil B, Flanagin A et al. Scientific Misconduct and Medical Journals. 2018;320(19):1985-1987 https://jamanetwork.com/journals/jama/fullarticle/2708590
2.George SL and Buyse M. Data fraud in clinical trials. Clin Investig (Lond). 2015; 5(2): 161–173.
3.Michalek AM, Hutson AD, Wicher CP et al. The Costs and Underappreciated Consequences of Research Misconduct: A Case Study. PLoS Med. 2018;7(8):e1000318. https://doi.org/10.1371/journal.pmed.1000318
4.Rutherford M. ICH E6(R2) and Data Integrity: Four Key Principles. Clinical Researcher. 2018 April;32(4):doi:10.14524/CR-18-4021. https://acrpnet.org/2018/04/17/ich-e6r2-data-integrity-four-key-principles/
5.https://www.pharmaceuticalonline.com/doc/an-analysis-of-fda-fy-drug-gmp-warning-letters-0003 Accessed on April 26, 2019
6.Moody LE and McMillan S. Maintaining data integrity in randomized clinical trials. Nur Res. 2002 Mar-Apr;51(2):129-33. https://www.ncbi.nlm.nih.gov/pubmed/11984384
7.http://firstclinical.com/fda-gcp/?show=MonitoringvAuditing&search=compliance&type=&page=1 Accessed on April 26, 2019
8.https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-good-clinical-practice-compliance-relation-trial-master-file-paper/electronic-content-management-archiving-audit-inspection-clinical-trials_en.pdf Accessed on April 26, 2019
9.Fleming TR, Sharples K, McCall J et al. Maintaining confidentiality of interim data to enhance trial integrity and credibility. Clin Trials. 2008;5(2):157-67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2703711/
10.Ellenberg SS. Protecting Clinical Trial Participants and Protecting Data Integrity: Are We Meeting the Challenges? PLoS Med. 2012 Jun;9(6):e1001234.
11.https://www.thefdagroup.com/thefdgroup-blog/conducting-data-integrity-audits-a-quick-guide Accessed on April 26, 2019.
The information contained on this article is intended solely to provide general guidance on matters of interest for the personal use of the reader, who accepts full responsibility for its use. Accordingly, the information on this article is provided with the understanding that the author(s) and publisher(s) are not herein engaged in rendering professional advice or services. As such, it should not be used as a substitute for consultation with a competent adviser. Before making any decision or taking any action, the reader should always consult a professional adviser relating to the relevant article posting.
While every attempt has been made to ensure that the information contained on this article has been obtained from reliable sources, Veeda Clinical Research is not responsible for any errors or omissions, or for the results obtained from the use of this information. All information on this article is provided “as is”, with no guarantee of completeness, accuracy, timeliness or of the results obtained from the use of this information, and without warranty of any kind, express or implied, including, but not limited to warranties of performance, merchantability and fitness for a particular purpose. Nothing herein shall to any extent substitute for the independent investigations and the sound technical and business judgment of the reader. In no event will Veeda Clinical Research, or its partners, employees or agents, be liable to the reader or anyone else for any decision made or action taken in reliance on the information on this article or for any consequential, special or similar damages, even if advised of the possibility of such damages. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, electronic, photocopying, recording or otherwise without the prior written permission of the publisher.
For information, contact us at:
Veeda Clinical Research Private Limited
Vedant Complex, Beside YMCA Club, S. G. Highway,
Vejalpur, Ahmedabad – 380 051,
Gujarat India.
Phone: +91-79-3001-3000
Fax: +91-79-3001-3010
Email: info@veedacr.com